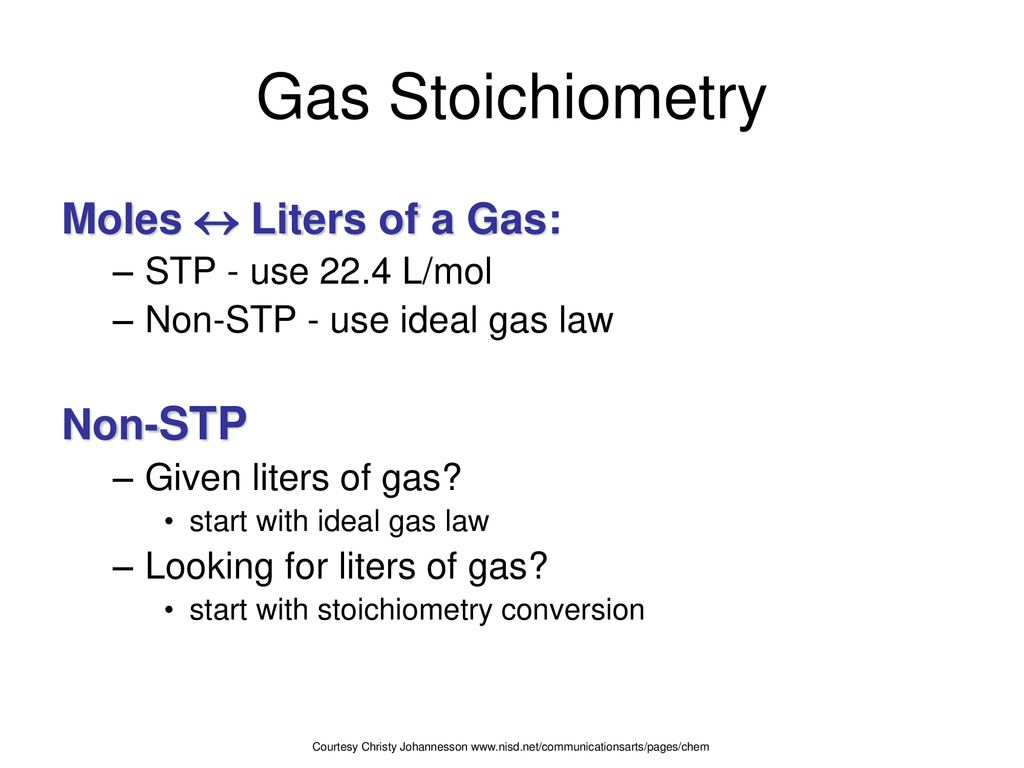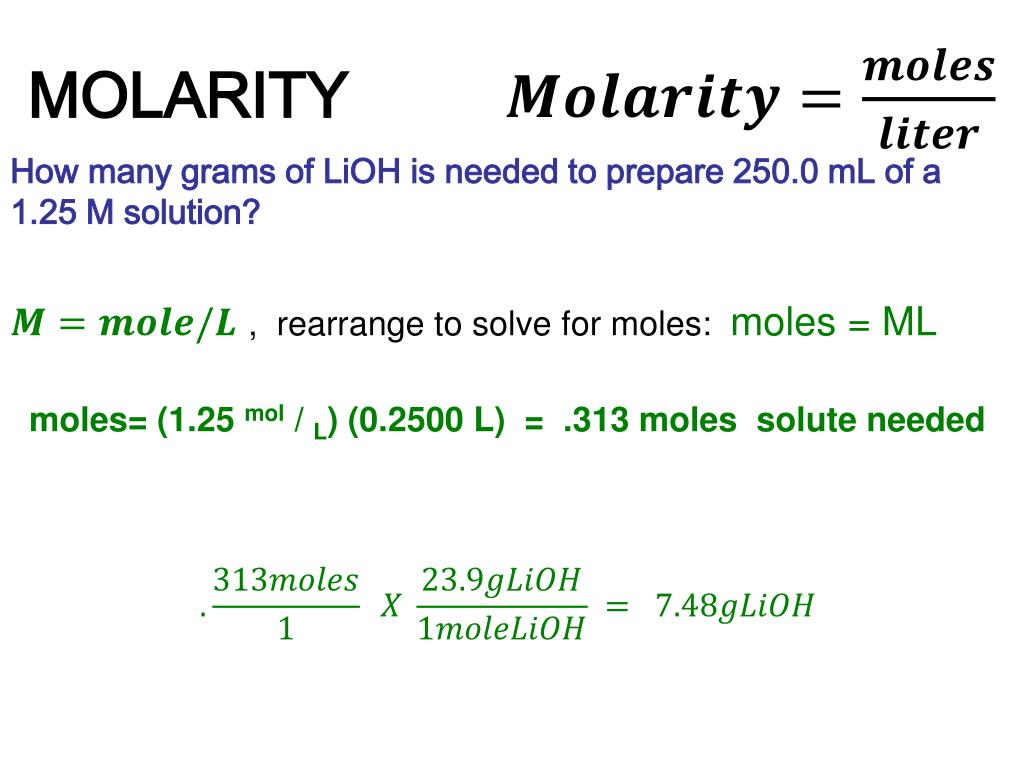
PPT - MOLARITY A measurement of the concentration of a solution Molarity (M) is equal to the moles of solute (n) per liter of PowerPoint Presentation - ID:1459925
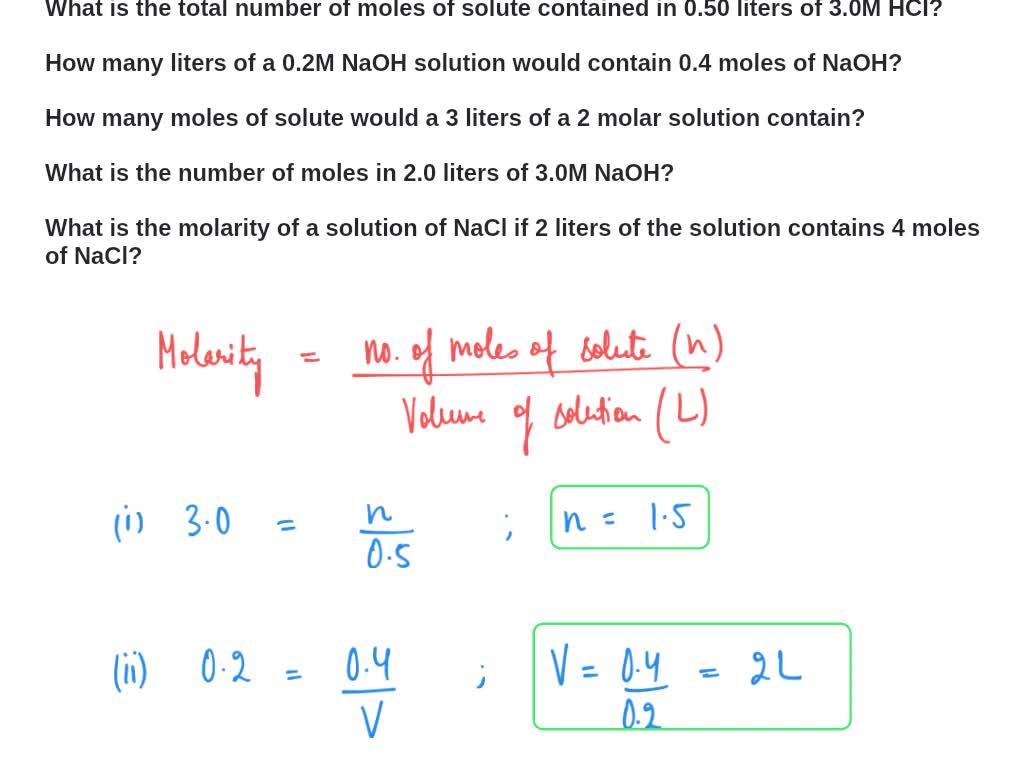
SOLVED: What is the total number of moles of solute contained in 0.50 liters of 3.0M HCl? How many liters of a 0.2M NaOH solution would contain 0.4 moles of NaOH? How

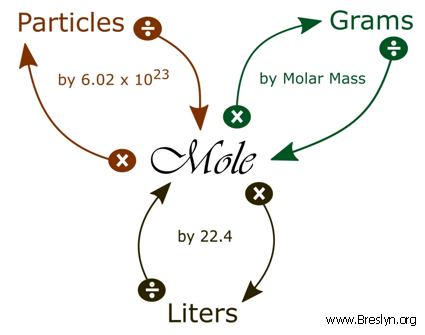
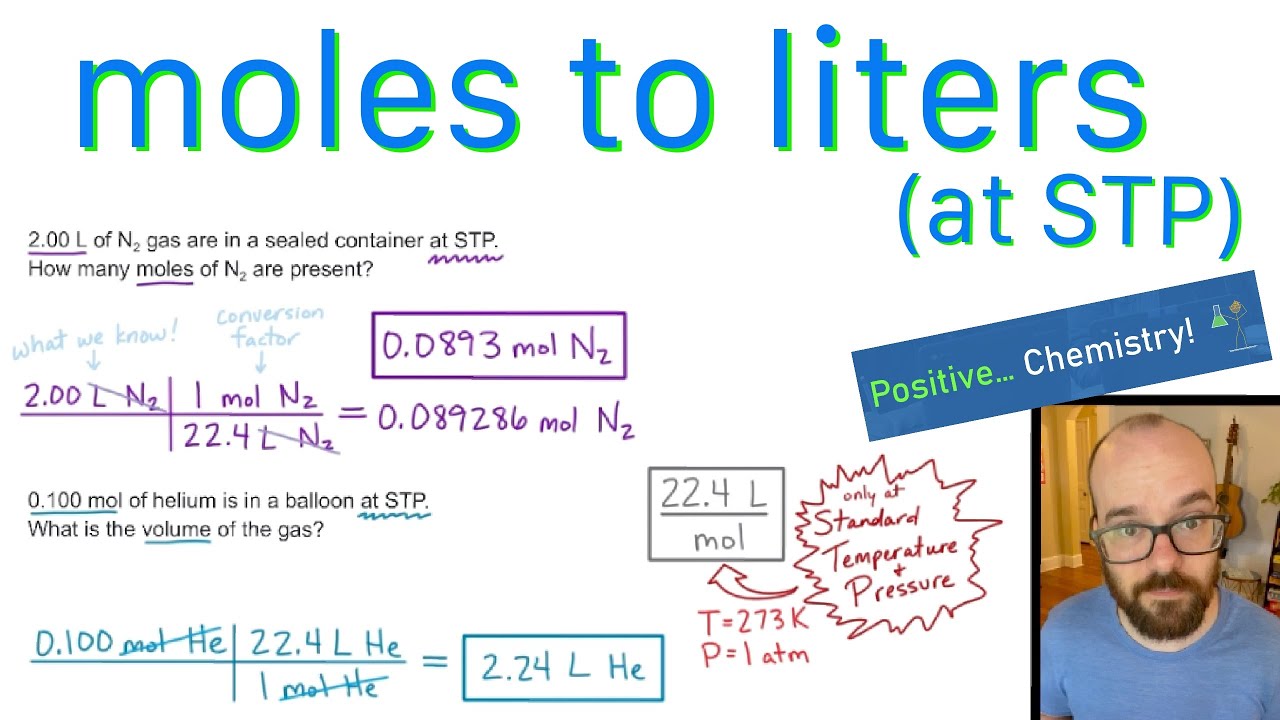
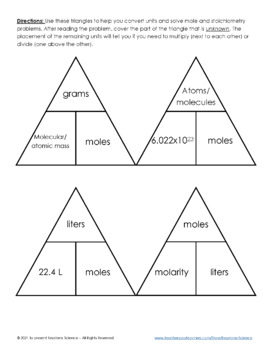
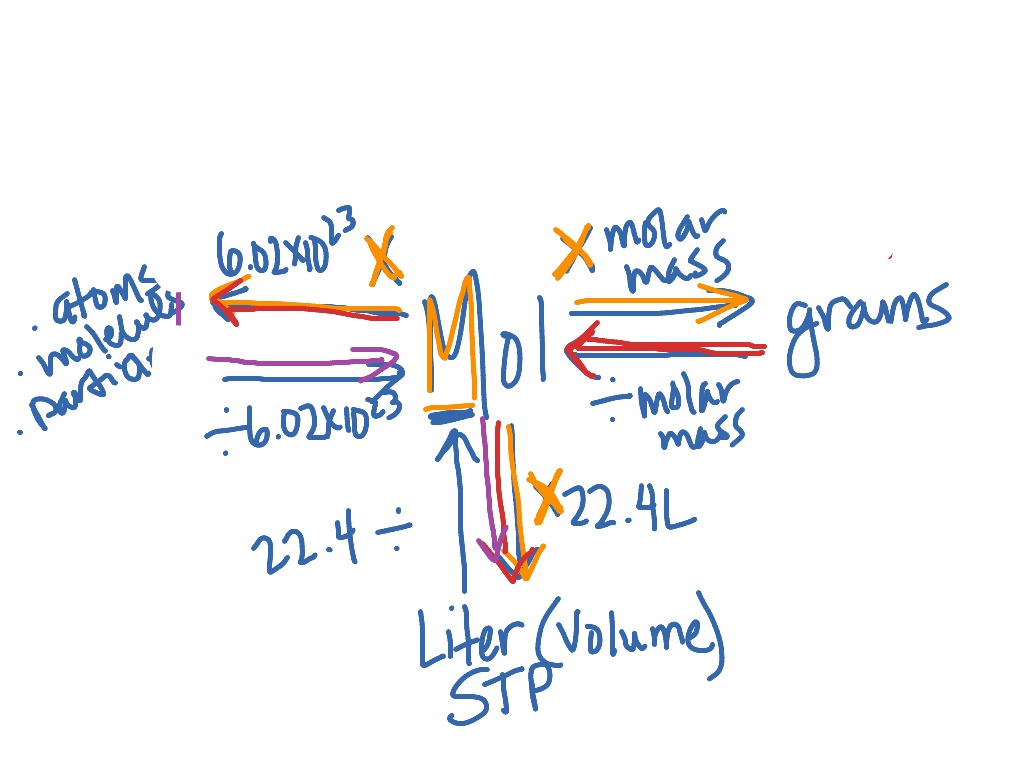
Answer Key Moles & Liters volume .docx - Moles & Liters Volume We've established that it's not practical to count atoms so we often need to use | Course Hero
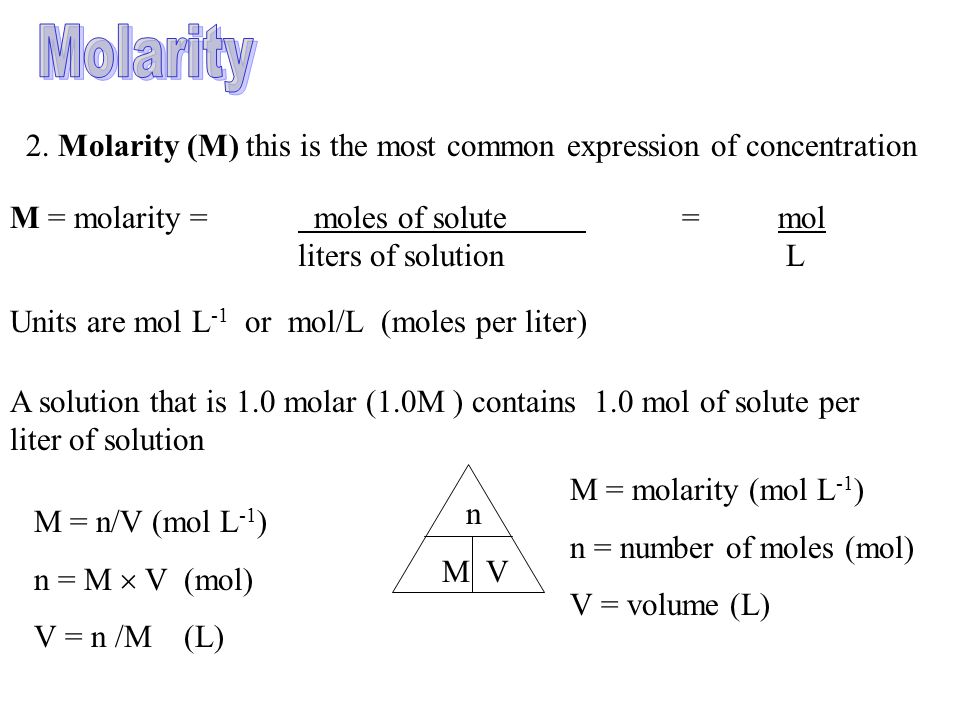
Molarity 2. Molarity (M) this is the most common expression of concentration M = molarity = moles of solute = mol liters of solution L Units are. - ppt download







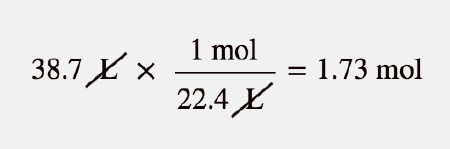



:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)