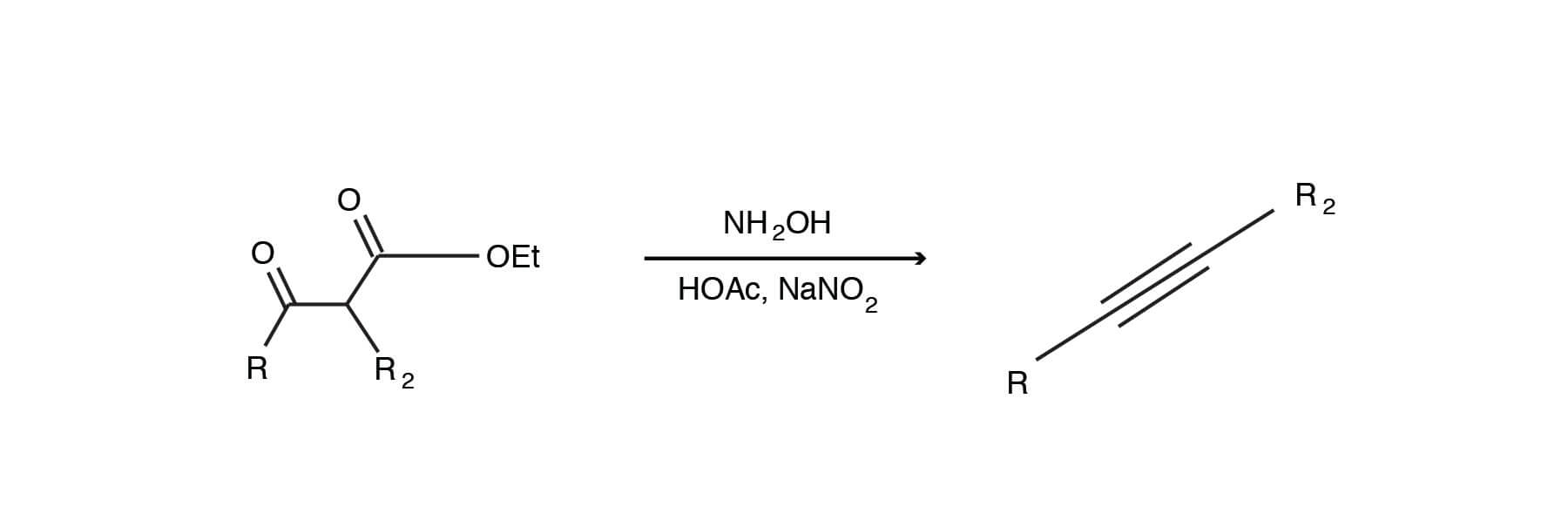
In the reaction between potassium carbonate and hydrochloric acid, 25 grams of salt were produced. If excess acid was available, how many grams of the carbonate were used? - Quora
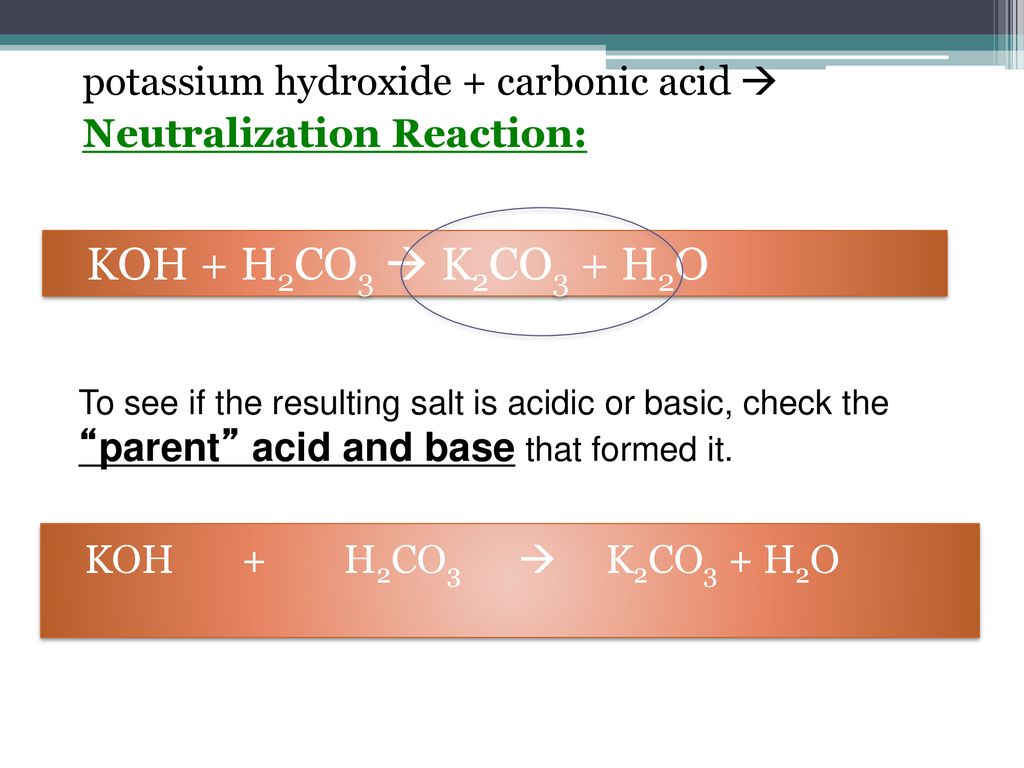
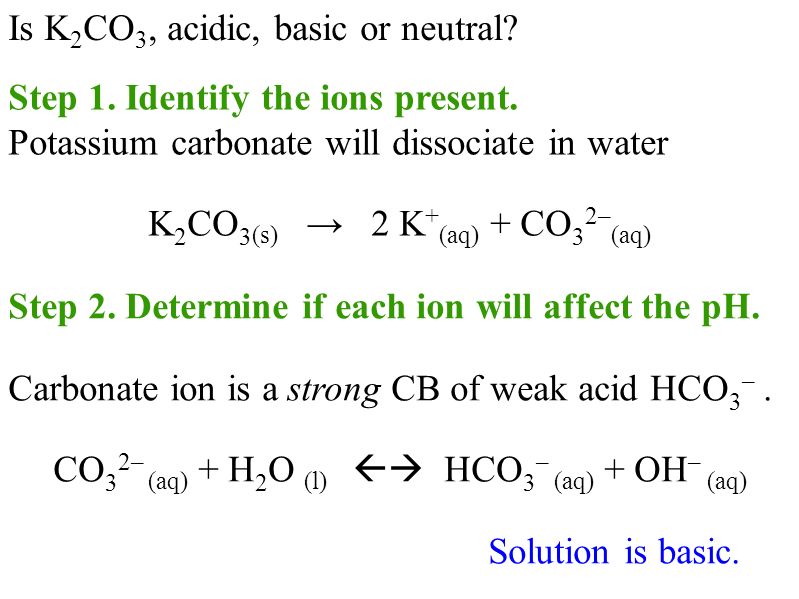
SOLVED: Select the salt from the list below which will produce a basic aqueous solution. Group of answer choices NaNO3 K2CO3 NH4Cl K2SO4
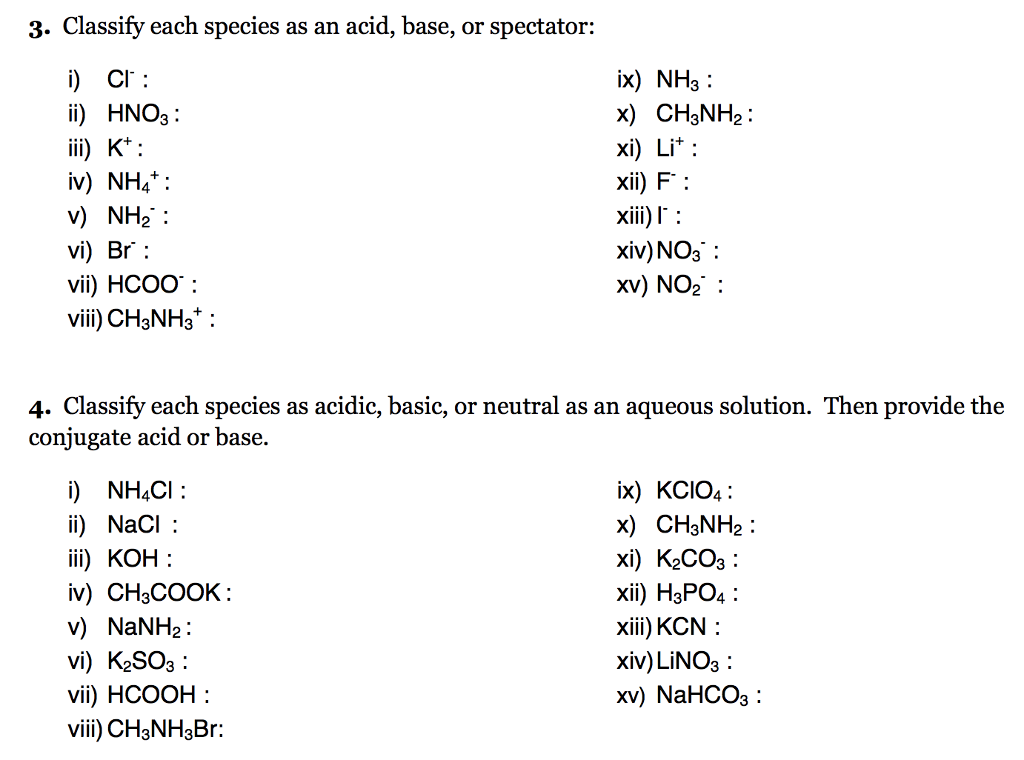
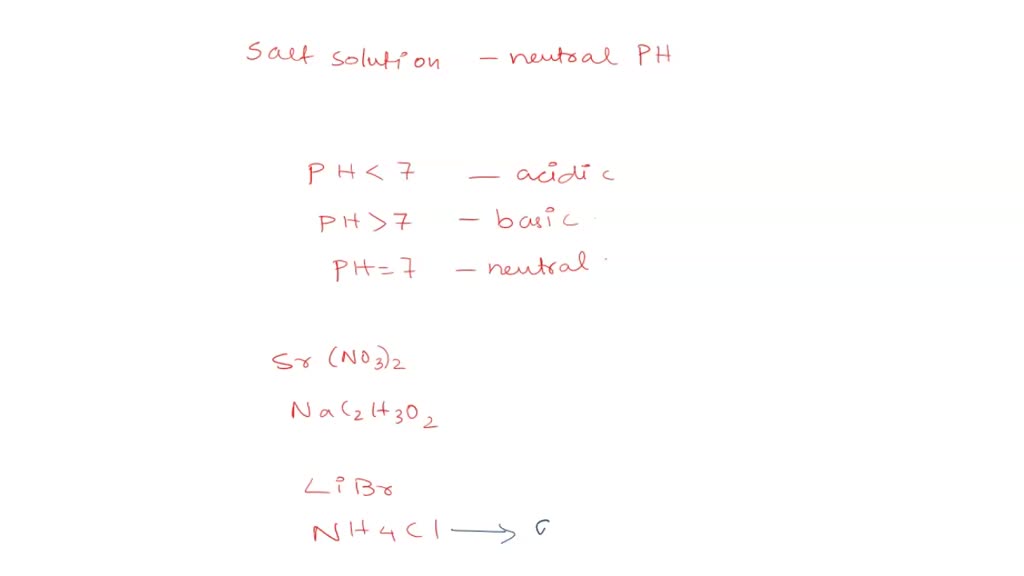
How would you determine is the following salts will from a solution that is acidic, basic, or pH neutral? CH3NH3CN, Fe(ClO4)3, K2CO3, CH3NH3CL, RbI | Socratic
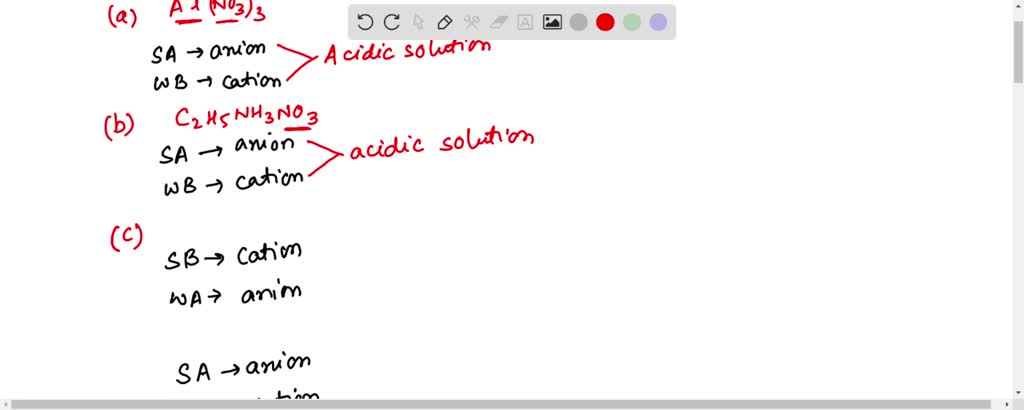
SOLVED: Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3 b. C2H5NH3NO3 c. K2CO3 d. RbI e. NH4ClO