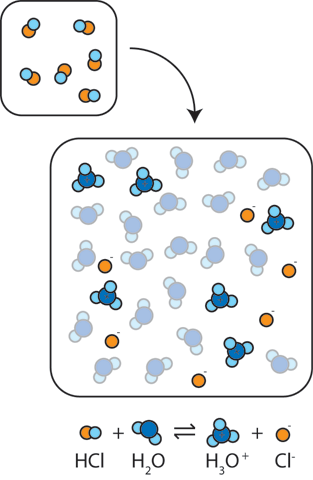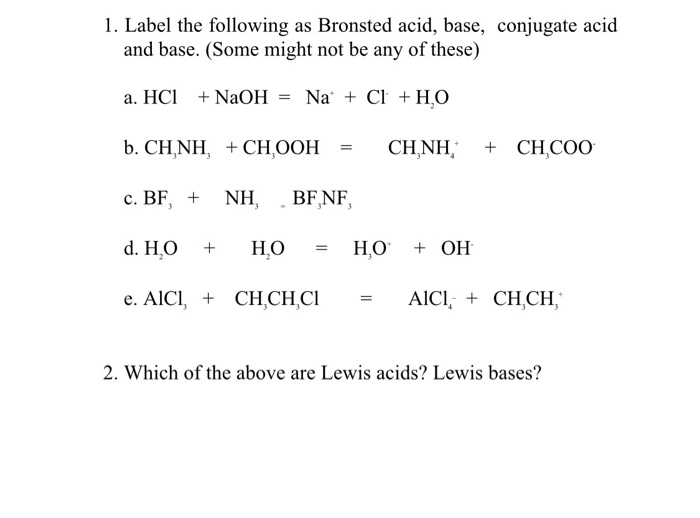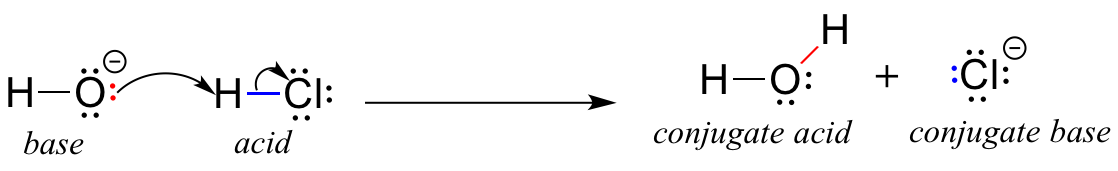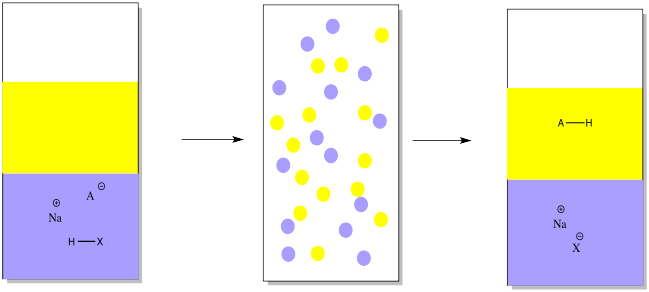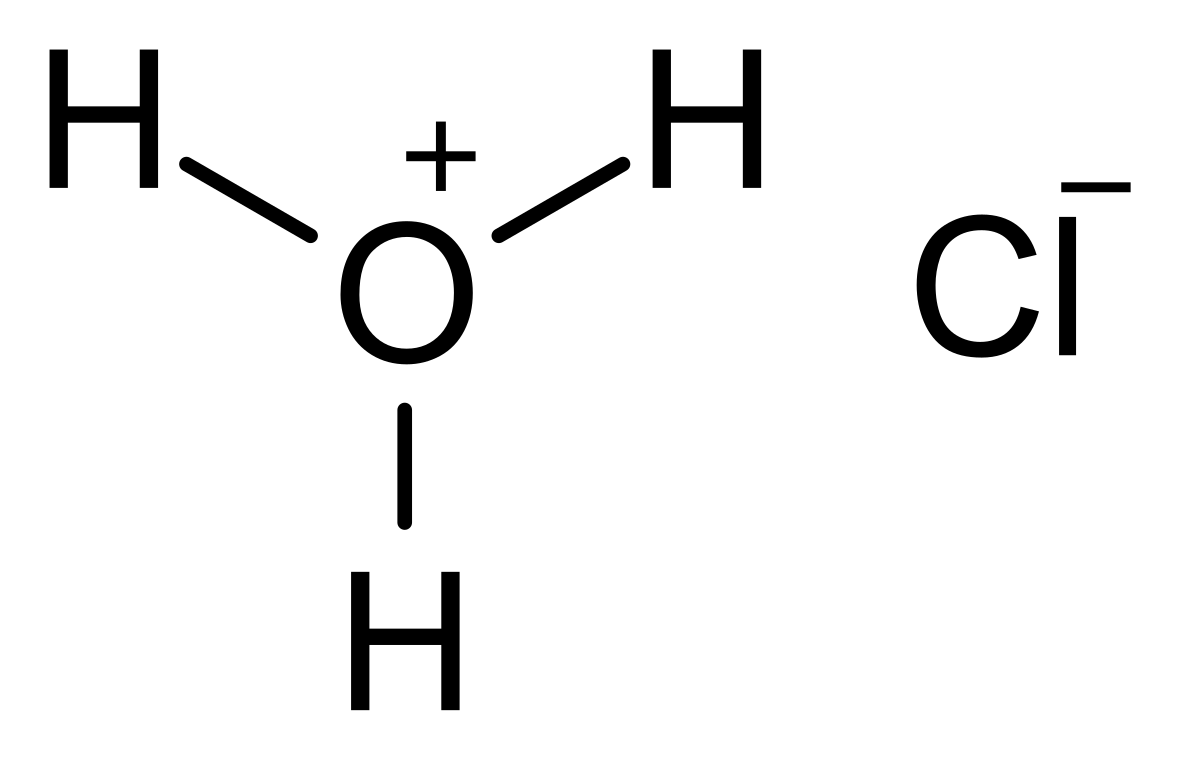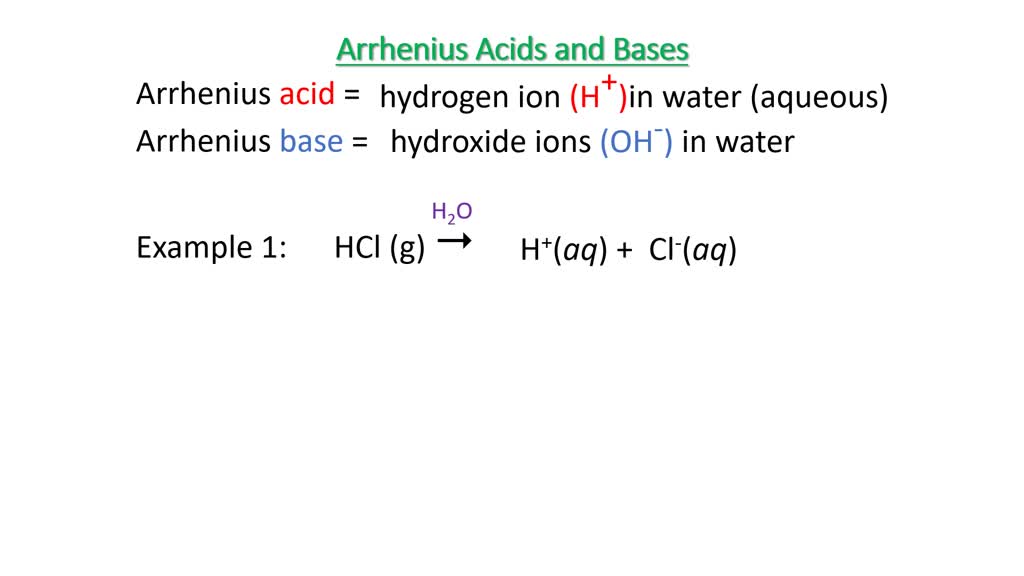
SOLVED:Write an equation showing how HCl (g) behaves as an Arrhenius acid when dissolved in water. Write an equation showing how NaOH(s) behaves as an Arrhenius base when dissolved in water.

Vector Illustration Of Electrolytic Dissociation Molecules Break Up Into Ions Chemical Containers With Acid Base And Salt Hcl Naoh And Nacl Stock Illustration - Download Image Now - iStock

Write the neutralization reaction between Hydrochloric acid HCI and sodium hydroxide NaOH, and write the equation for this process.

Hydrogen Chloride vs. Hydrochloric Acid | Formula, Properties & Examples - Video & Lesson Transcript | Study.com







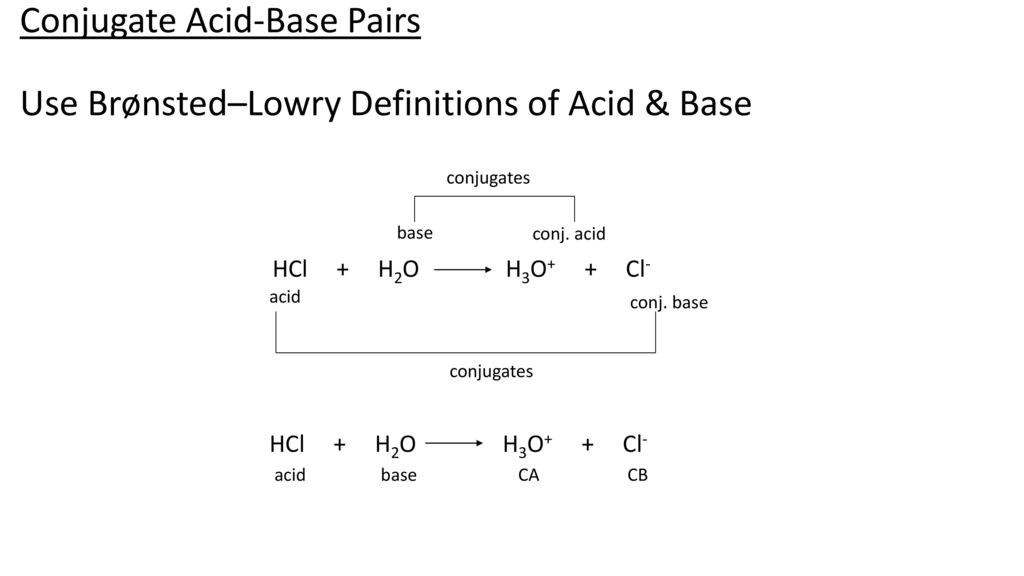

:max_bytes(150000):strip_icc()/what-is-muriatic-acid-608510_final-49086a2dccff45e9a643d22de405c8a4.png)