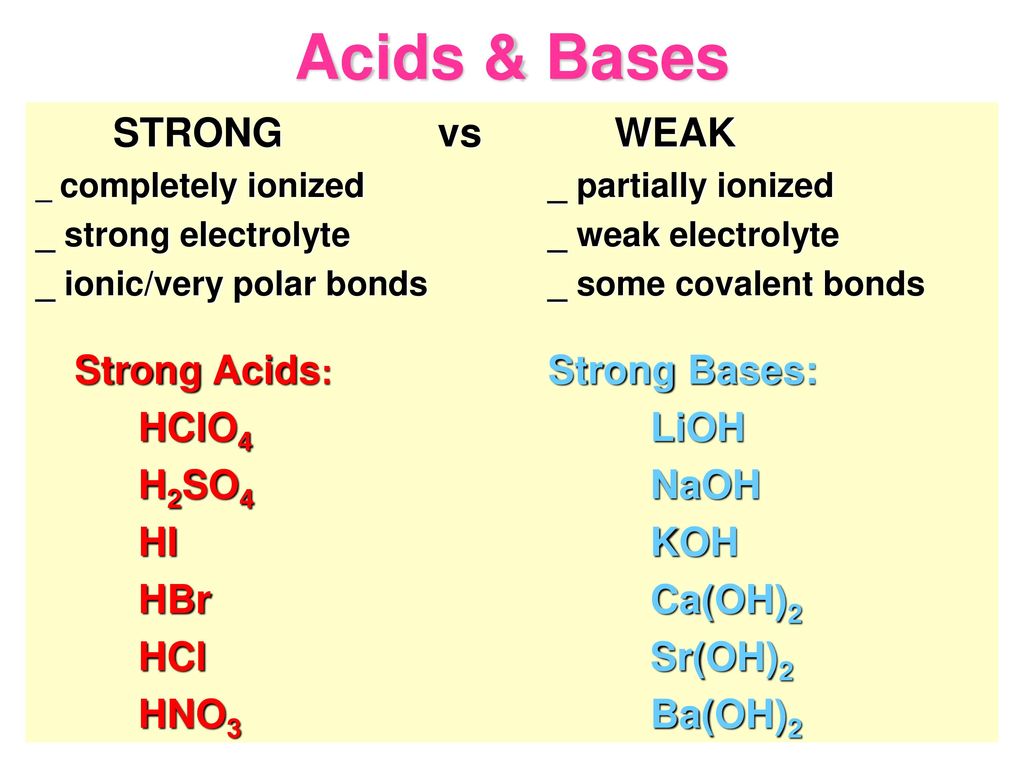
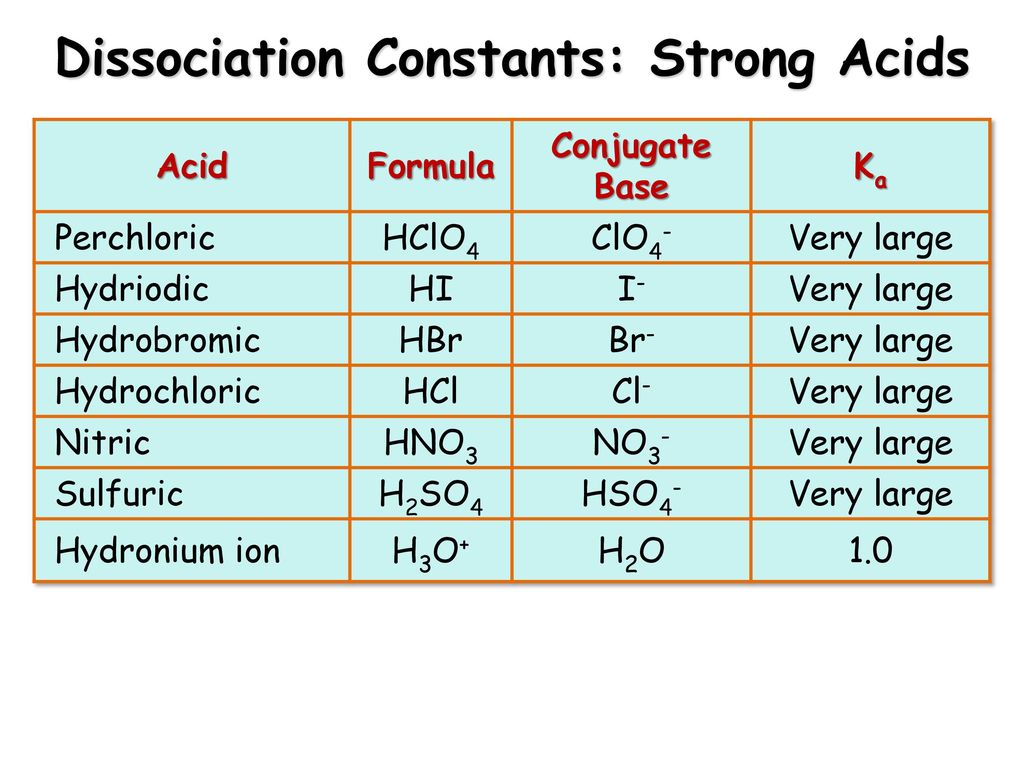
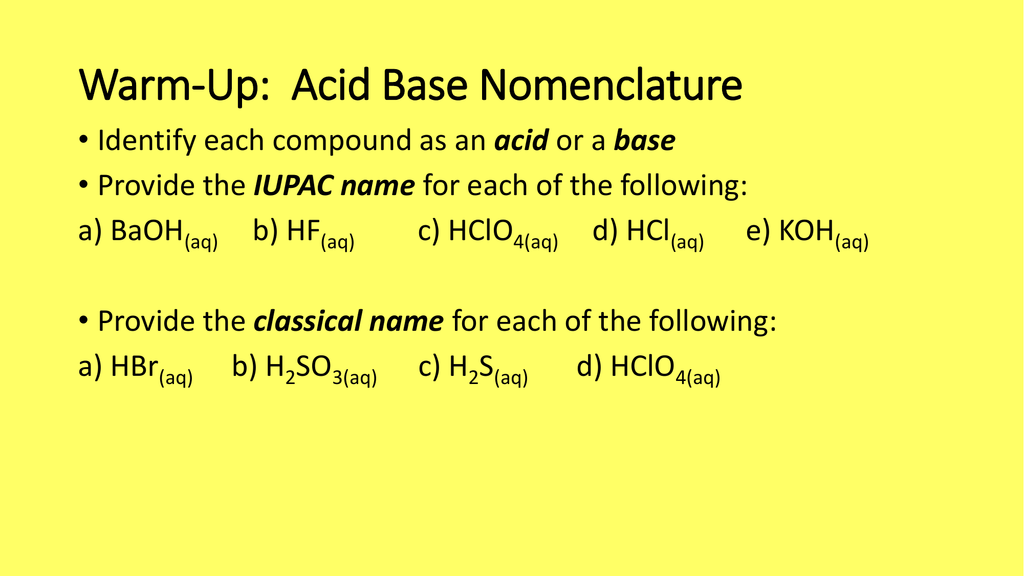
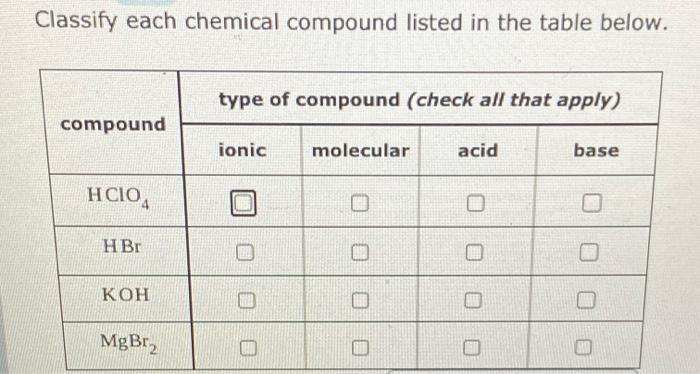
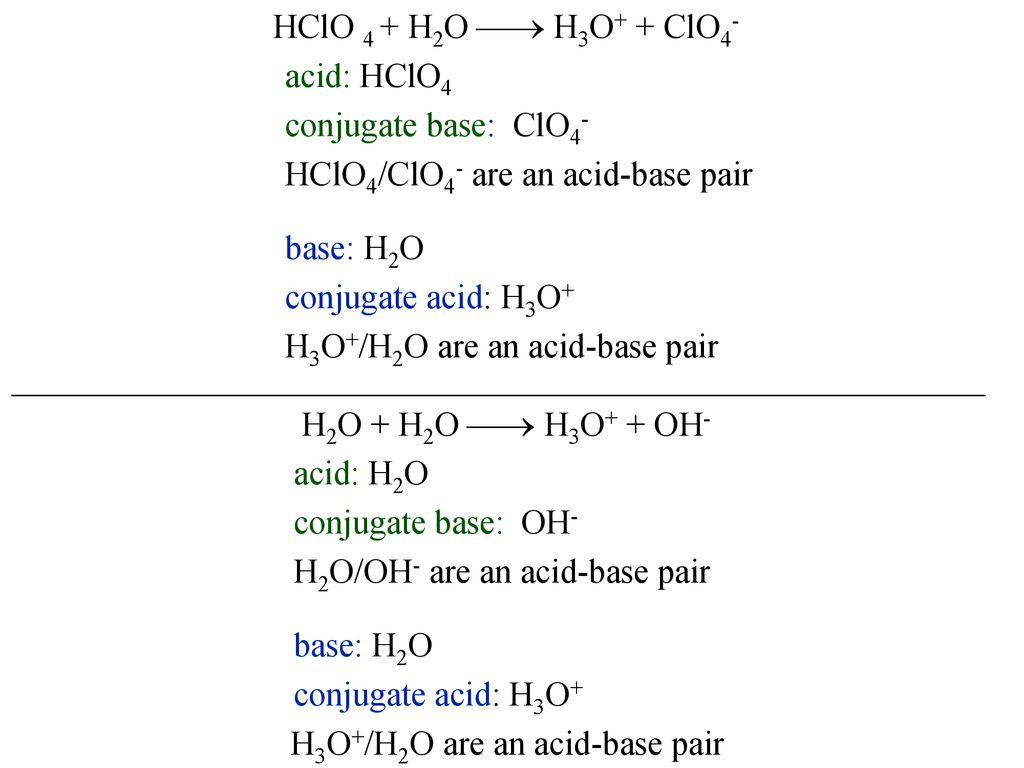
What is meant by the conjugate acid-base pair? Find the conjugate acid//base for the following species: HNO(2), CN^(Θ), HClO(4), F^(Θ), overset(Θ)(O)H, CO(3)^(2-), and S^(2-)

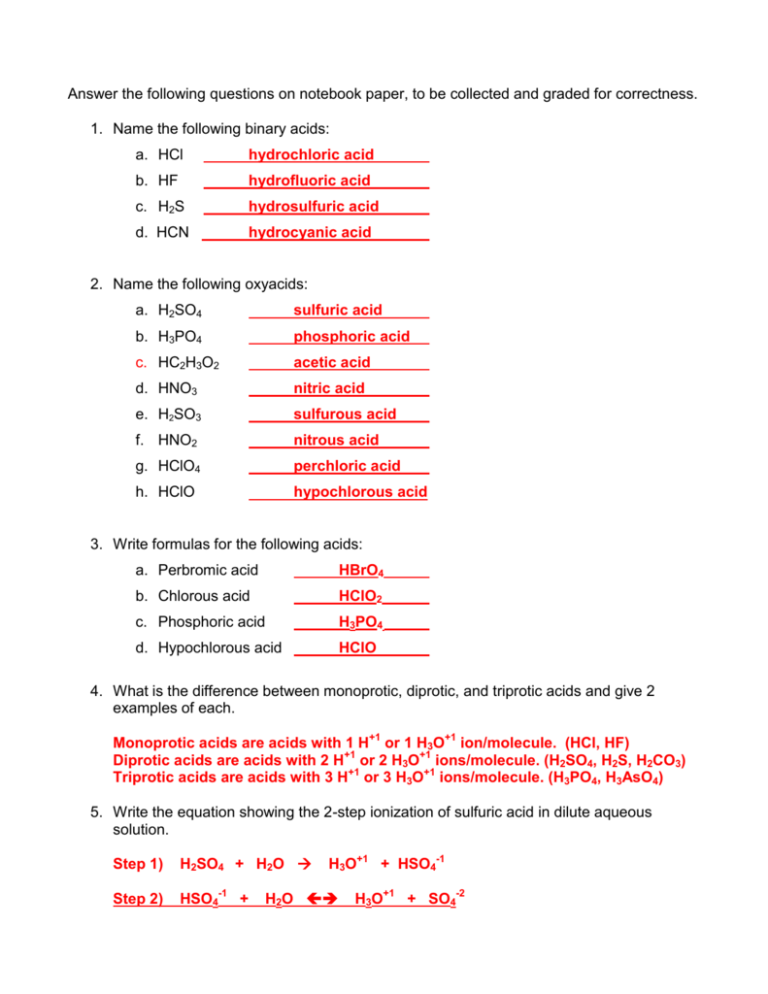
What is meant by conjugate acid-base pair? Find the conjugate acid/base for the following species.. - YouTube

See: Write an equation to show how perchloric acid, HClO4, reacts with water. Include states of matter in - Brainly.com

SOLVED:Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. (a) HClO4 (b) Mg(OH)2 (

SOLVED: Enter a chemical equation for HClO4(aq) showing how it is an acid or a base according to the Arrhenius definition. Consider that strong acids and bases dissociate completely. Express your answer
![In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora](https://qph.cf2.quoracdn.net/main-qimg-db330d0184802e0ca001e7ca26960b0e.webp)
In the following equation, what is acting as the Bronsted acid: [math]HClO_4 + H_2O\to ClO_4^- + H_3O^+[/math]? - Quora