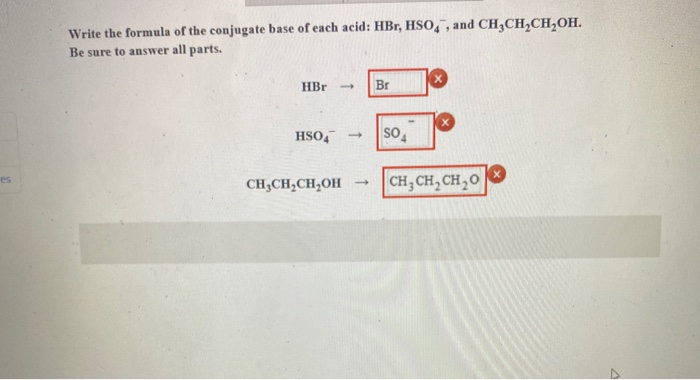
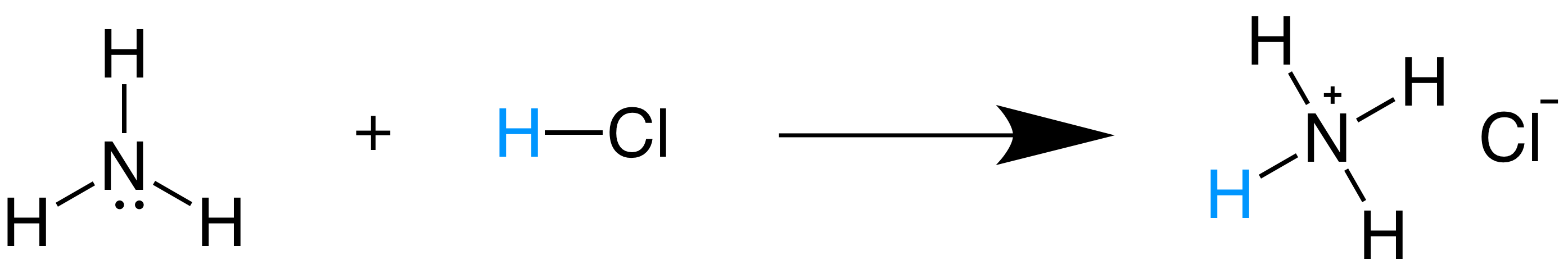HL Acids and Bases. Strength of Acids/Bases Strong Acids (100% ionized or dissociated) – HCl – HBr – HI – HNO 3 – H 2 SO 4 – HClO 4 – HClO 3 Strong bases. - ppt download

Existing literature and proposed circuits (a, b, c). HBR, DSHBR, HBRJT... | Download Scientific Diagram
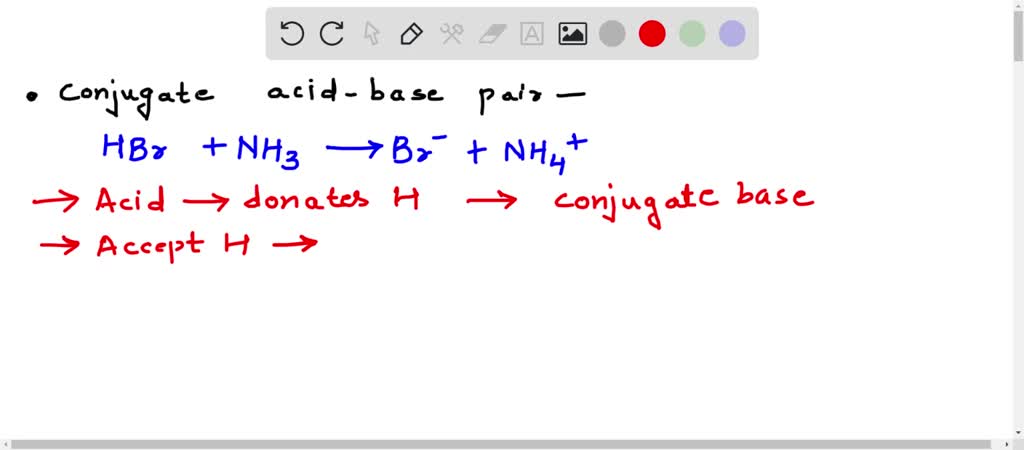
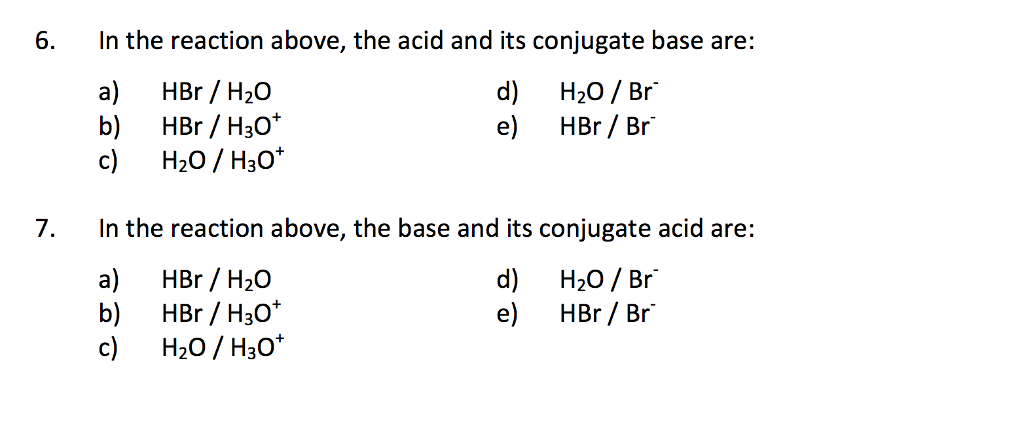
SOLVED: Identify the conjugate acid-base pairs in the reaction shown below HBr + NH3 Br + NH4 acid; chemPad Help Gianke conjugate base: chemPad Help Girekt base: chemPad Help Creke conjugate acid:

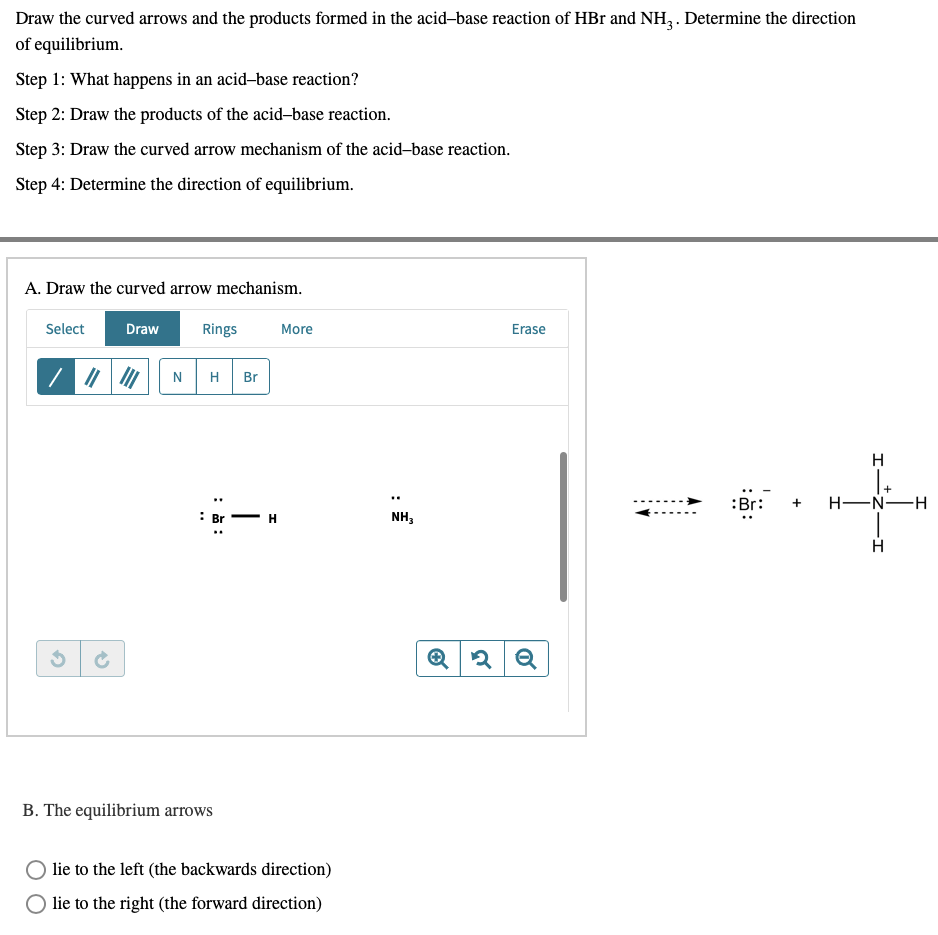
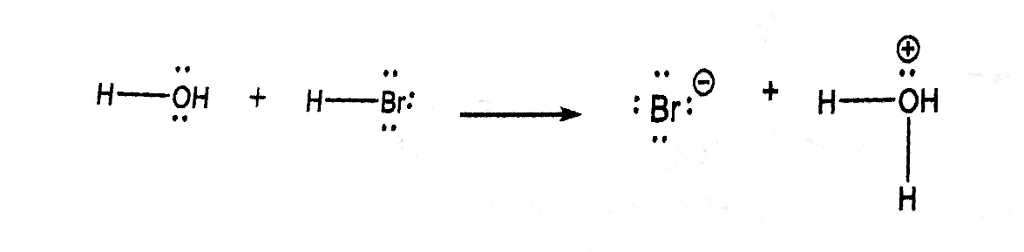
PHILIPPINE CHEMISTRY PROFESSIONALS BOARD EXAM REVIEWER - Which one of the following mechanistically depicts the acid-base reaction that occurs when hydrobromic acid(HBr) is added to methanol (CH4O)? Please refer to attached image.

1 Introduction to Acids and Bases The earliest definition was given by Arrhenius: An acid contains a hydrogen atom and dissolves in water to form a hydrogen. - ppt download