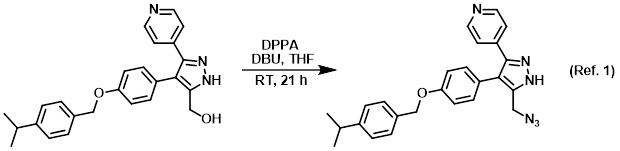
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/large/ol-2015-02398j_0002.jpeg)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/large/ol-2015-02398j_0001.jpeg)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters

DBU‐Promoted Intramolecular Crossed Aldol Reaction: A Facile Access to Indane‐Fused Pyrrolidine - Yang - 2019 - European Journal of Organic Chemistry - Wiley Online Library

DBU‐Promoted Nucleophilic Activation of Carbonic Acid Diesters - Carafa - 2011 - European Journal of Organic Chemistry - Wiley Online Library
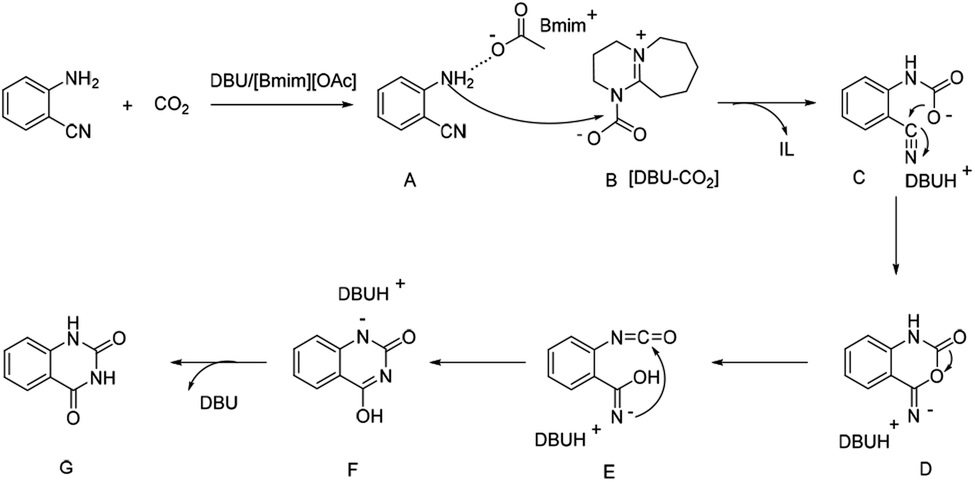
DBU coupled ionic liquid-catalyzed efficient synthesis of quinazolinones from CO 2 and 2-aminobenzonitriles under mild conditions - RSC Advances (RSC Publishing) DOI:10.1039/D0RA00194E
![The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters](https://pubs.acs.org/cms/10.1021/acs.orglett.5b02398/asset/images/medium/ol-2015-02398j_0006.gif)
The Dual Role of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) in the Synthesis of Terminal Aryl- and Styryl-Acetylenes via Umpolung Reactivity | Organic Letters
![Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/da071cf8-eee0-4e2f-8e22-5b6f6a0f91e7/adsc201601279-fig-5004-m.jpg)
Controlled Reactivity of 1,8‐Diazabicyclo[5.4.0]undec‐7‐ene (DBU) in the Selective Synthesis of 1‐(Bromoethynyl)arenes - Krishna Moodapelly - 2017 - Advanced Synthesis & Catalysis - Wiley Online Library

Structure of the PILs' precursors: DBU base (a) and the three acids... | Download Scientific Diagram
![Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S2052411022024750-c9qo00602h-ga.jpg)
Mechanistic investigation-inspired activation mode of DBU and the function of the α-diazo group in the reaction of the α-amino ketone compound and EDA: [DBU-H]+-DMF-H2O and α-diazo as strong N-terminal nucleophiles - ScienceDirect
Proposed mechanisms for the DBU catalyzed urea-synthesis reaction from... | Download Scientific Diagram
![1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science](https://www.eurekaselect.com/images/graphical-abstract/coc/19/9/0005D.gif)
1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU): A Versatile Reagent in Organic Synthesis | Bentham Science

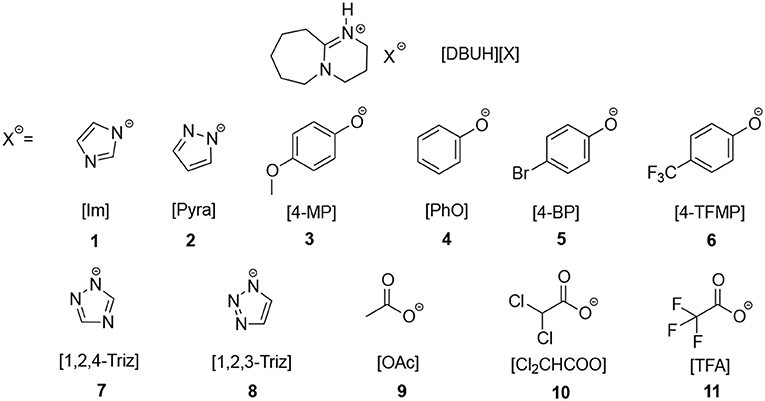
SciELO - Brasil - Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry and DFT Studies of Catalyst Role Mechanistic Investigation of DBU-Based Ionic Liquids for Aza-Michael Reaction: Mass Spectrometry

DBU, 1,8-diazabicyclo 5.4.0 undec-7-ene, is a base in elimination reactions. Which N atom is more basic in DBU? Explain your choice. | Homework.Study.com
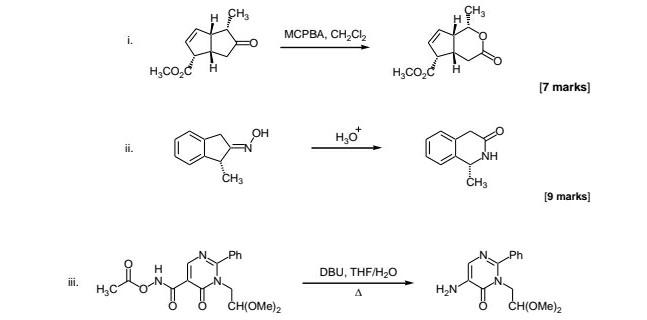




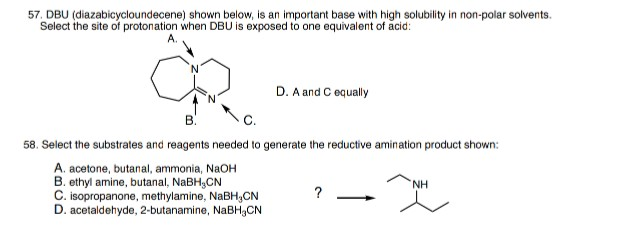

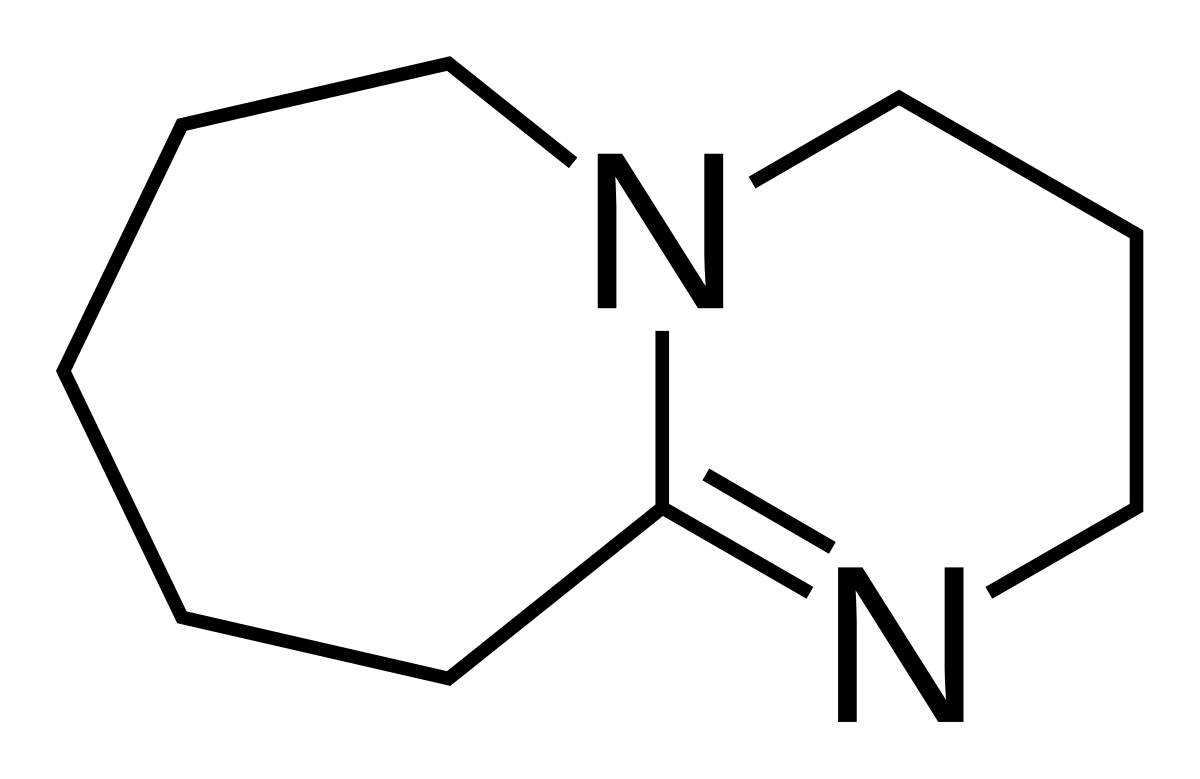
![DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube DBU [1,8-Diazabicyclo[5,4,0]undec-7-ene] - An Overview - YouTube](https://i.ytimg.com/vi/fu67cE_ydew/maxresdefault.jpg)