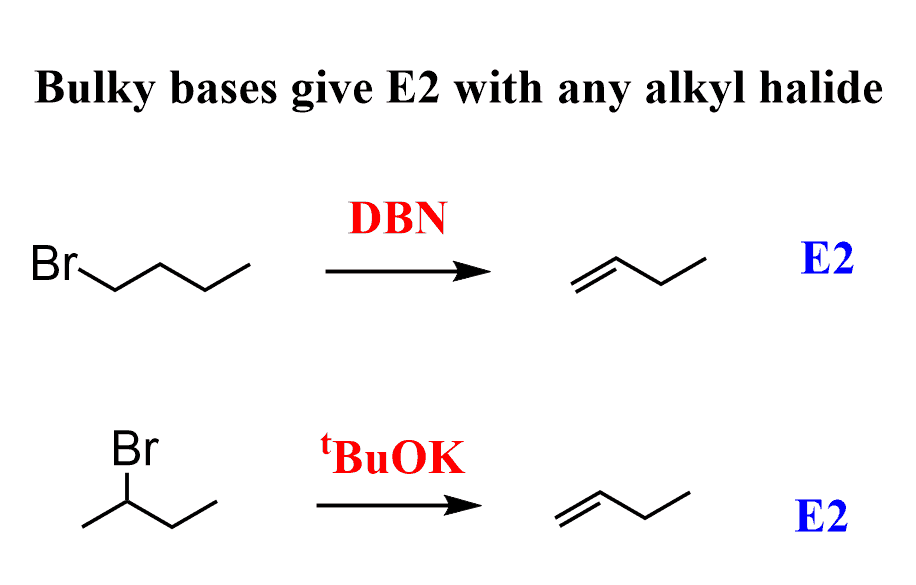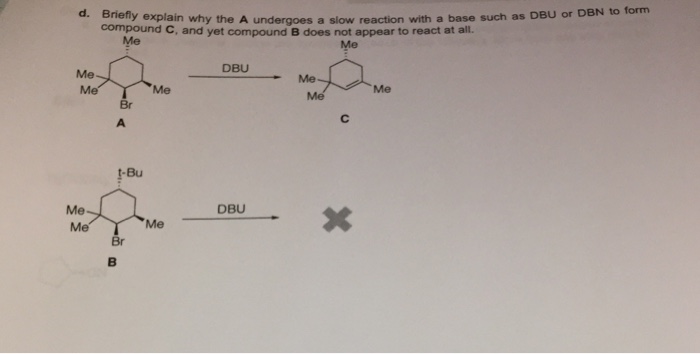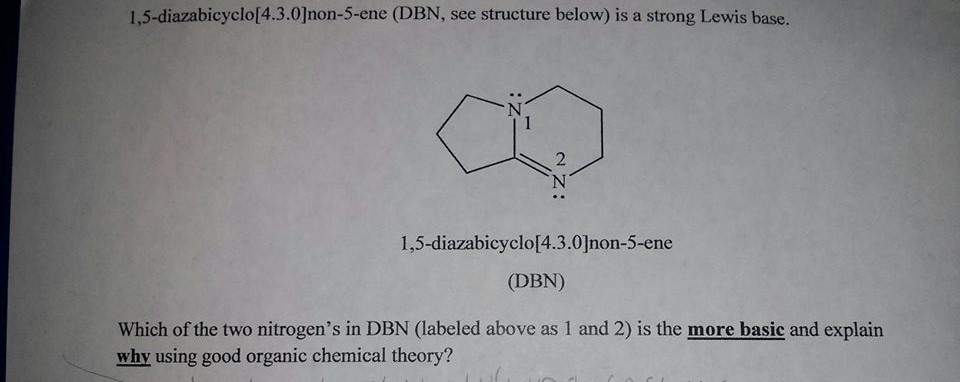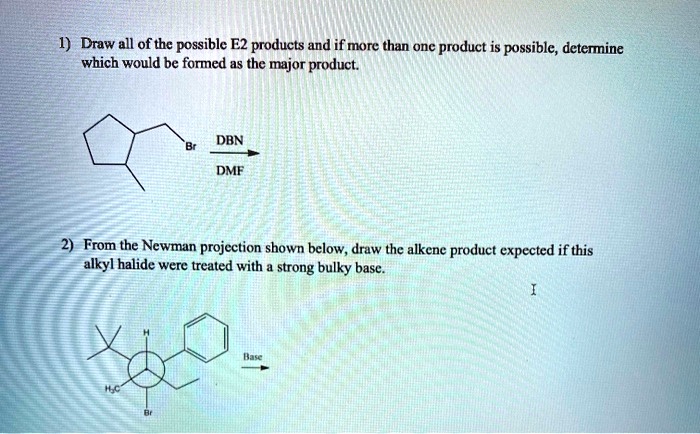
SOLVED: Draw all of the possible E2 products and if more than one product is possible; determine which would be formed as the major product: DBN DMF From the Newman projection shown
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen

The basic structures of the restricted Boltzmann machine and the DBN model. | Download Scientific Diagram
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen
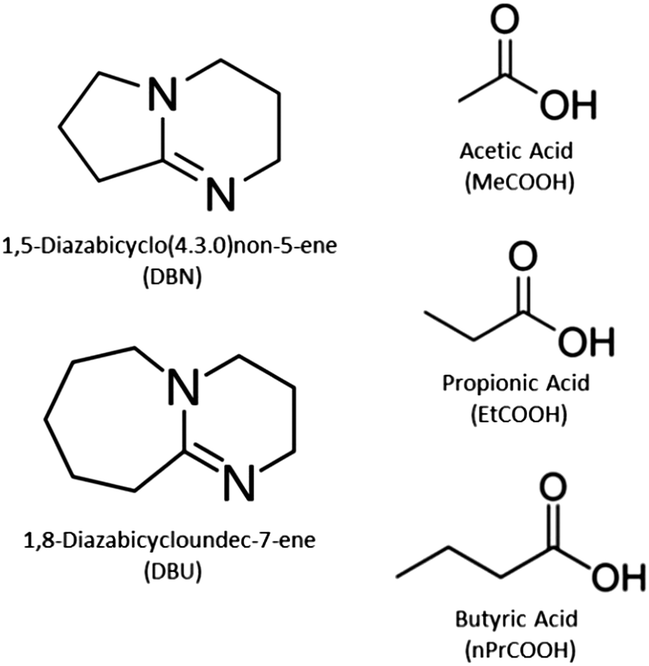
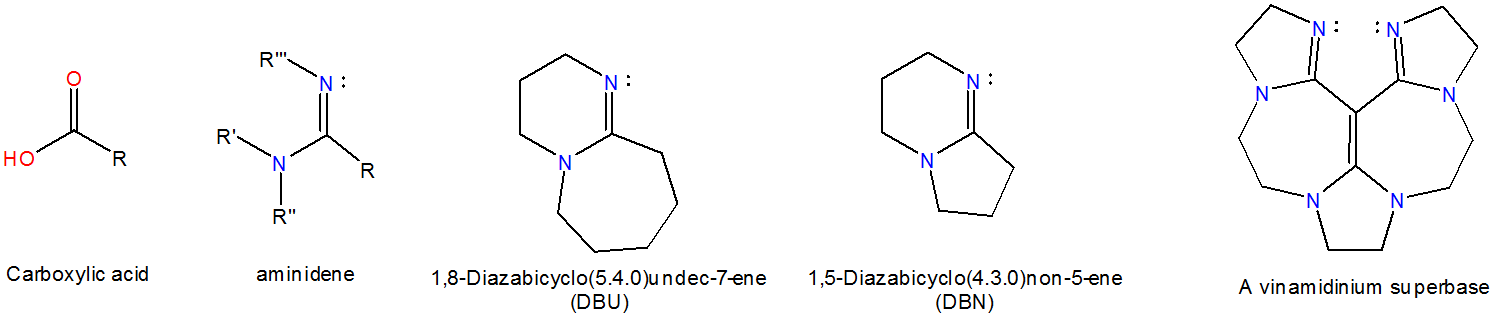
Vaporization of protic ionic liquids derived from organic superbases and short carboxylic acids - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C7CP02023F

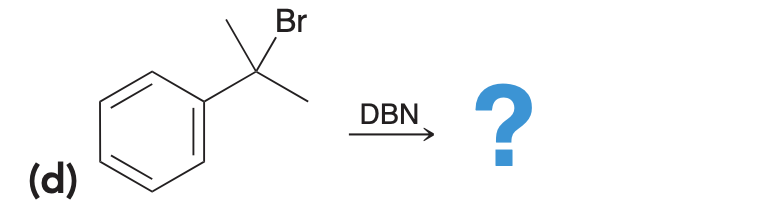
DBN is a bicyclic compound which is used as base. What is the major product in the following reaction?

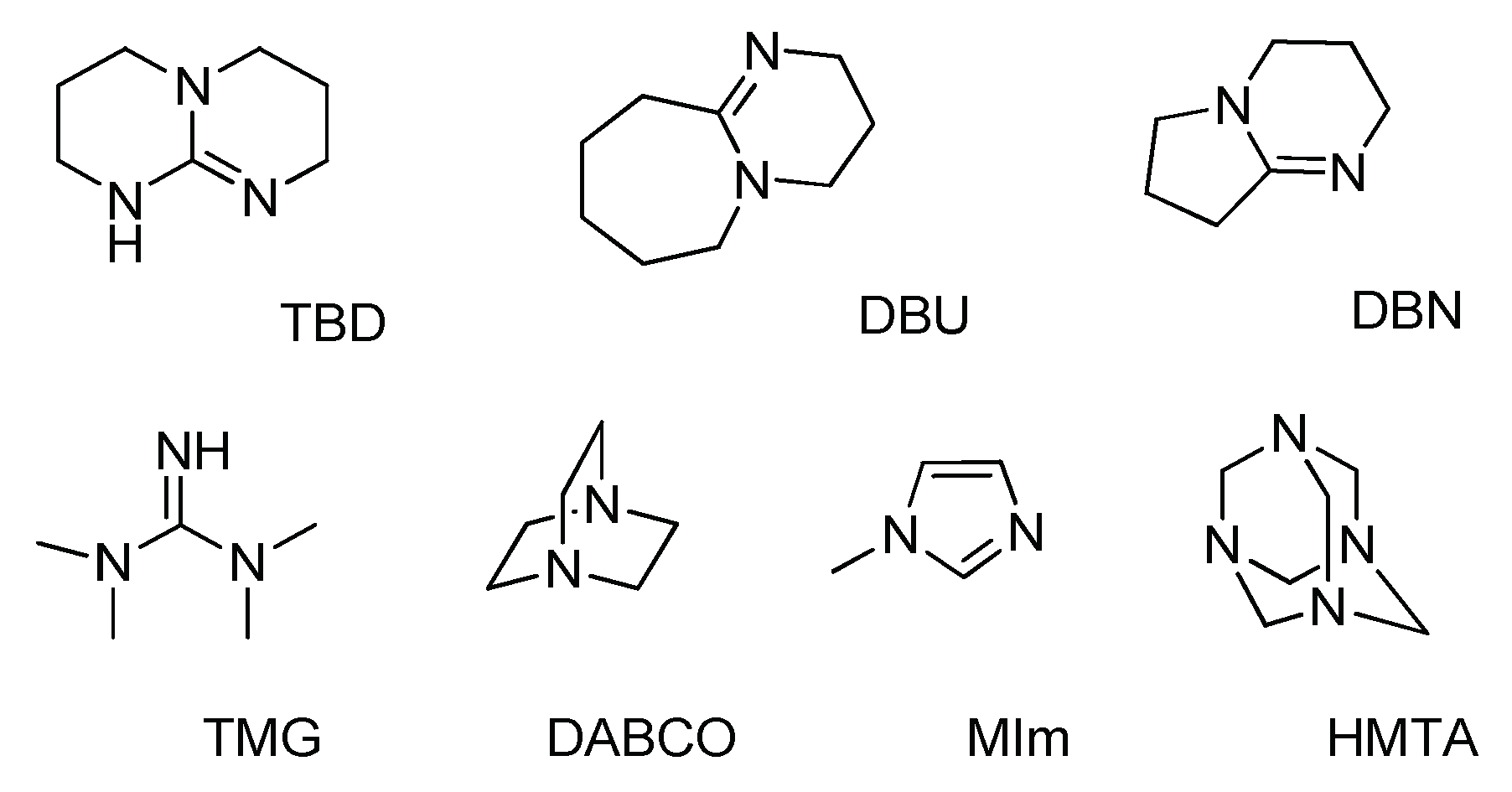
Indicate which nitrogen in each of these bases is the one most likely to be protonated? Are they more basic than regular amines? Choose the correct options:
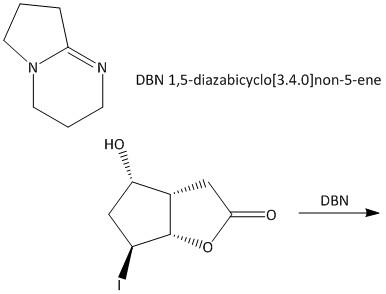
DBN is a bicyclic compound which is used as a base. What is the major product in the following reaction?\n \n \n \n \n A. \n \n \n \n \n B. \n \

DBN is a bicyclic compound which is used as base. What is the major product in the following reaction?